Molecule Shapes
|
|
Original Sim and Translations |
About
Topics
- Molecules
- VSEPR
- Lone Pairs
- Bonds
Description
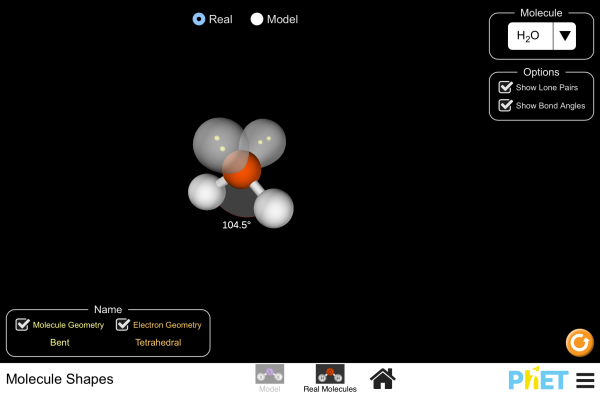
Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!
Sample Learning Goals
- Recognize that molecule geometry is due to repulsions between electron groups.
- Recognize the difference between electron and molecular geometry.
- Name molecule and electron geometries for molecules with up to six electron groups surrounding a central atom.
- Compare bond angle predictions from the VSEPR-based model to real molecules.
- Describe how lone pairs affect bond angles in real molecules.
Keywords
For Teachers
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
Browse more activities.
Translations
Related Simulations
Software Requirements
| Windows 7+ | Mac OS 10.7+ | iPad and iPad Mini with iOS | Chromebook with Chrome OS |
|---|---|---|---|
|
Microsoft Edge and Internet Explorer 11, latest version of Firefox, latest version of Chrome.
|
Safari 9+, latest version of Firefox, latest version of Chrome.
|
Latest version of Safari
|
Latest version of Chrome
|
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|