Isotopes and Atomic Mass
|
|
|
Back to HTML5 Version |
About
Topics
- Isotopes
- Atomic Mass
Description
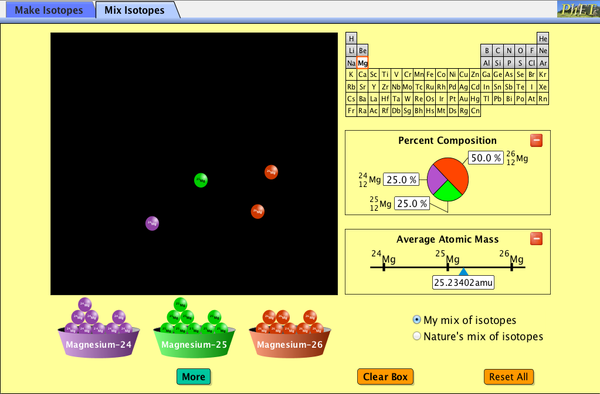
Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
Sample Learning Goals
- Define “isotope” using mass number, atomic number, number of protons, neutrons and electrons.
- Given information about an element, find the mass and name of an isotope.
- Give evidence to support or dispute: “In nature, the chance of finding one isotope of an element is the same for all isotopes.”
- Find the average atomic mass of an element given the abundance and mass of its isotopes.
- Predict how the mass and name of an isotope will change given a change in the number of protons, neutrons or electrons.
- Predict how the average atomic mass of an element changes given a change in the abundance of its isotopes.
Keywords
For Teachers
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
Translations
Related Simulations
Software Requirements
| Windows | Macintosh | Linux |
|---|---|---|
|
Microsoft Windows XP/Vista/7/8.1/10 Latest version of Java
|
OS X 10.9.5 or later Latest version of Java
|
Latest version of Java
|
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|
|