Sugar and Salt Solutions
|
About
Topics
- Solutions
- Ionic
- Covalent
Description
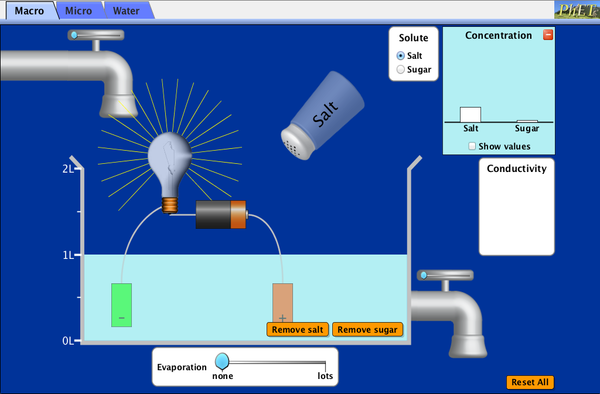
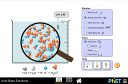
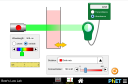
What happens when sugar and salt are added to water? Pour in sugar, shake in salt, and evaporate water to see the effects on concentration and conductivity. Zoom in to see how different sugar and salt compounds dissolve. Zoom in again to explore the role of water.
Sample Learning Goals
- Use pictures and proportional reasoning to explain changes in concentration
- Draw what happens at the molecular level when compounds dissolve in water
- Identify if a compound is a salt or sugar by macroscopic observations or microscopic representations.
- Explain how using combinations of solutes changes solution characteristics or not.
- Use observations to explain ways concentration of a solute can change.
- Describe ways the formula, macroscopic observations, or microscopic representations of a compound indicates if the bonding is ionic or covalent.
Keywords
For Teachers
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
Translations
Related Simulations
Software Requirements
| Windows | Macintosh | Linux |
|---|---|---|
|
Microsoft Windows XP/Vista/7/8.1/10 Latest version of Java
|
OS X 10.9.5 or later Latest version of Java
|
Latest version of Java
|
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|
|