Salts & Solubility
|
About
Topics
- Solubility
- Salt
- Solutions
- Chemical Equilibrium
- Saturation
- Chemical Formula
- Ksp
- Le Chatelier's Principle
Description
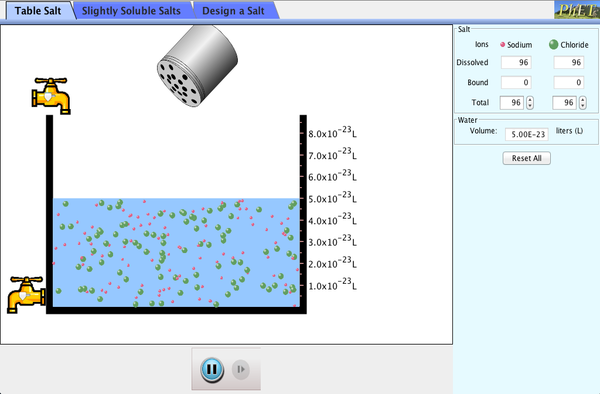
Add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Compare the number of ions in solution for highly soluble NaCl to other slightly soluble salts. Relate the charges on ions to the number of ions in the formula of a salt. Calculate Ksp values.
Sample Learning Goals
- Rank the solubility of different salts.
- Determine the ratio of anions and cations that create a neutral compound.
- Calculate the molarity of saturated solutions, and Ksp values.
Keywords
For Teachers
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
Translations
Related Simulations
Software Requirements
| Windows | Macintosh | Linux |
|---|---|---|
|
Microsoft Windows XP/Vista/7/8.1/10 Latest version of Java
|
OS X 10.9.5 or later Latest version of Java
|
Latest version of Java
|
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|